Octopus trial advances with first Welsh site
The world’s first multi-arm, multi-stage (MAMS) platform trial to transform and discover treatments for MS has opened a new site in Cardiff.
We're excited to announce a milestone for the first ever mega-trial into treatments for progressive MS.
The Octopus trial, funded by us, has now opened a new site at the University Hospital of Wales in Cardiff. And has recruited its first participant to the site!
The Welsh site is one of up to 30 sites that will eventually open around the UK.
Octopus is being led by researchers from the Queen Square MS Centre and MRC Clinical Trials Unit at University College London (UCL).
We’re delighted to see the Octopus trial site opening in Cardiff. More than 5,600 people live with MS in Wales – over 130,000 throughout the UK. And there are thousands with progressive forms who have nothing to stop their MS getting worse. We won’t stop until we have treatments that transform the lives of everyone with MS.Shelley Elgin, Country Director, MS Society Cymru
What is the Octopus trial?
Octopus is a groundbreaking clinical trial for progressive multiple sclerosis (MS) that's unique worldwide. It allows us to test several different drugs at once using a single control group.
It also combines what would typically be two separate trials into one. It does that by using MRI scans to give us an early idea of whether a drug might be effective, well before we can see the effect on disability. This approach offers us the flexibility to drop drugs that don't show promise and include new ones as we discover them.
The Octopus team aims to enroll a minimum of 1,200 people with progressive MS over the next six years. This will provide a chance for people who’ve never participated in a trial before to take part.
Who is the first trial participant at the Welsh site?
The first participant to join the Octopus trial in Cardiff is Lisa Haines, 55-year-old former banking customer service advisor. She was diagnosed with primary progressive MS in 2006, three months after having her second child. In 2009 she had to medically retire because of severe fatigue.
She registered for the trial through the UK MS Register:
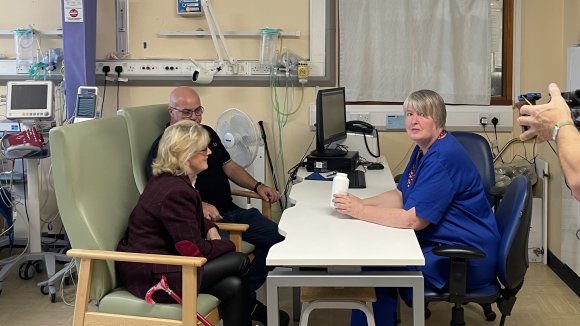
I felt brilliant being given a place on the trial. I couldn’t believe it! There is nothing else for people with primary progressive MS, so I’ve got nothing to lose. And if it doesn’t help me then it could only forward research for someone in the future. MS is such a horrible condition but I live in hope!Lisa Haines who lives with primary progressive MS
Helping people from the UK and beyond
Dr Emma Tallantyre, Octopus recruitment Lead and Principal Investigator at University Hospital of Wales, has been working in MS trials for 15 years.
She says:
Octopus is a landmark trial, one of the first of its kind in MS. […] This is such an exciting opportunity for people, like Lisa, who currently have no or limited treatment options to have something that could be disease modifying.
And it’s great to feel that, despite being a relatively small country, the UK might be responsible for moving things forward for people with MS on a global scale.
Dr Emma Tallantyre, Octopus recruitment Lead
Anyone who has primary or secondary progressive MS in the UK can register their interest through the UK MS Register.
Register your interest in Octopus
When you register, you’ll be asked where you live. This is so the closest trial sites can contact you when they start recruiting. For most people, this won’t happen for quite a while. That's because trial sites are still getting set up and over 1,600 people have already registered their interest.