What can we learn from studying HSCT in the real world?
Most HSCT data comes from clinical trials. But last week, a new study was published looking at the effects of HSCT in a real-world healthcare setting.
We’re learning more and more about the possible benefits of HSCT in MS, and who’s likely to experience them. Most evidence is from clinical trials that tightly control who takes part.
Last week, experts from Imperial College London including both neurologists and haematologists, reported similar effects when people received HSCT as part of their regular MS care.
What did the researchers do?
They did what’s called an observational study. Unlike in clinical trials, there was no comparison group of people who didn’t have HSCT. This means we can’t predict what would have happened to the people involved if they hadn’t had the treatment. So the researchers can’t draw definitive conclusions about the role HSCT played, or how HSCT compares to other treatments.
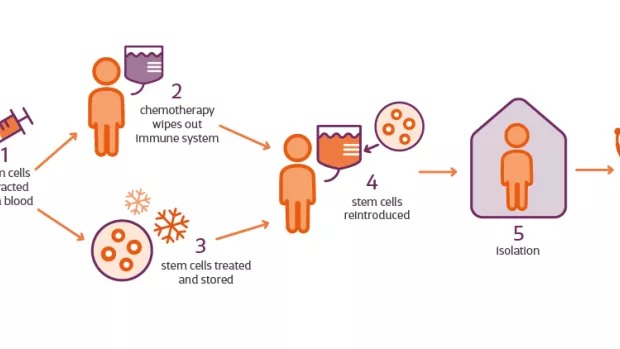
The team looked back at data from 120 people who had HSCT at two hospitals in London between 2012 and 2019. The data comes from people’s regular hospital visits between six months and four years after HSCT. They investigated whether the number of relapses and new lesions changed after HSCT. And whether people’s disability got worse or not.
Who took part?
To decide who could take part, at first a group of clinicians would come together to agree HSCT was suitable for someone. Later on, formal criteria were developed to make sure they were including people with inflammatory active MS. This is because the current understanding is that HSCT targets inflammation. But there was still more flexibility than in trials.
Almost everyone (90%) had MRI inflammation. So they showed new active lesions on an MRI scan. 58 people had relapsing MS, 40 had secondary progressive MS and 22 had primary progressive MS.
What did the results show?
- Relapses and lesions
Across the whole group, rates of relapses and new MRI lesions were significantly lower in the years following HSCT than the years before it.
Four years after HSCT, 87% of people were free from relapses. Only people with relapsing MS experienced relapses following HSCT, which makes sense because relapse rates were much higher in this group before HSCT too.
85% were free from new MRI lesions. And there were no differences between relapsing and progressive MS.
- Disability
In the short-term (one year after HSCT), as a group, people with relapsing MS showed an improvement in disability, but people with progressive MS showed slightly worsening disability.
65% of people followed up at four years showed no worsening of disability since having HSCT. The rates were similar for people with progressive and relapsing MS.
The researchers also found certain things were associated with disability worsening - having high levels of a particular protein in your blood or symptoms of infection with the Epstein-Barr virus after HSCT.
- Safety
Almost everyone (90%) had a least one early complication, like fever, diarrhoea, severe vomiting or admission to intensive care. And sadly, three people died, reminding us of the serious risks HSCT comes with. Two of these people had progressive MS and one had relapsing MS. All three had an EDSS score of 6.5.
The researchers say their observations support previous research suggesting a range of factors can together contribute to making someone at greater risk from HSCT. These include having progressive MS, more advanced disability and living with certain other health conditions.
What does this mean for people with MS?
These observations from a small group of people in a real-world healthcare setting, are similar to clinical trial results. Many, but not all, of those followed for four years remained free from relapses and/or new MRI lesions. And a majority remained free of disability-worsening.
Because this was an observational study, the researchers say their findings can’t tell us for sure whether and how people with progressive MS benefit from HSCT. But they say their observations do support further research to explore the impact of HSCT on active progressive MS.
A next step could be to directly compare HSCT with the new DMTs approved for progressive MS (ocrelizumab and siponimod). Similar trials are already ongoing in relapsing MS.
Professors Richard Nicholas and Paolo Muraro, who led the research, said: "Looking at the effects of HSCT outside the tight confines of a clinical trial can expand the horizon into who might benefit. This uncontrolled study raises the question - could HSCT be a safe and effective option for people living with inflammatory progressive MS?
"Our observations strengthen the case for research directly comparing HSCT with the DMTs that have recently been licensed for people with progressive MS with evidence of active inflammatory disease - siponimod and ocrelizumab."