Positive results for clinical trial in progressive MS
Researchers in the US have proclaimed their initial test of ibudilast for MS a success.
This drug is already used as a medicine for stroke and asthma. It's now being investigated for its potential to protect nerves in MS and other neurological conditions.
On target
As part of the phase 2 clinical trial, 255 people with primary and secondary progressive MS were given a pill with ibudilast or a placebo. This included some people already on the Disease Modifying Therapies (DMTs) glatiramer acetate and interferon beta.
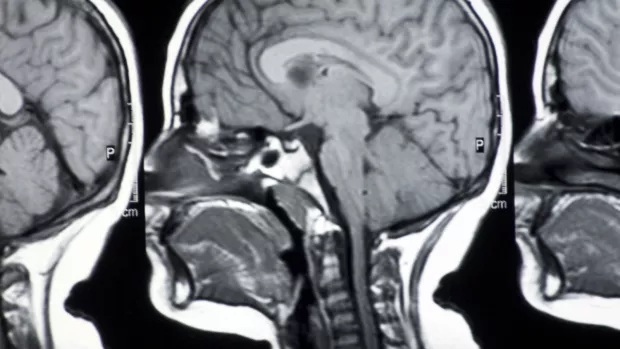
The study, called SPRINT-MS, found that people taking the drug had a significant reduction in their rate of brain atrophy (shrinkage) compared to those on placebo. This was measured using MRI results.
They also found that the drug was safe and well tolerated by people.
Breaking news
Full details of the results will be presented at the European Committee for Treatment and Research in MS (ECTRIMS) conference on Saturday morning.
Commenting on the outcome the lead researcher, Dr. Robert Fox, said: “This is an encouraging step forward in the development of treatments for progressive MS, which has historically been very difficult to treat.”
Badly needed
Dr Susan Kohlhaas, our Director of Research, said: “We are all impatient for treatments for progressive MS, and it’s great to see these positive results for ibudilast, a drug that’s already used for stroke and asthma. In spite of how far research has come, people are still left without options once told their condition has advanced.
"This trial and ongoing research in the UK are vital if we’re to change that. We’re now keen to see a final stage phase 3 trial that can confirm exactly how effective and safe ibudilast is as a treatment for MS.”