7 things you need to know about HSCT
Autologous haematopoietic stem cell transplantation (HSCT) is a recognised treatment for some forms of MS.
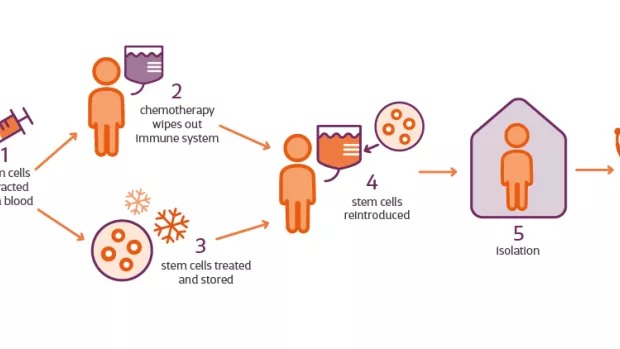
1. What is HSCT?
In MS the immune system mistakenly attacks the protective layer around nerves, called myelin. HSCT (sometimes referred to as AHSCT) is a procedure that aims to reset the faulty immune system to stop this happening.
Haematopoietic stem cells are taken from your bone marrow or blood, then a chemotherapy drug is used to wipe out your immune system. The stem cells are then reintroduced into your blood, where they grow a new immune system that will hopefully no longer attack your myelin.
2. Who can benefit from HSCT?
HSCT tackles the immune system – as do all existing disease modifying therapies (DMTs). So we tend to see the best results in those whose MS symptoms are being caused by inflammation and immune attacks (shown by relapses or on an MRI).
Studies into HSCT have found that HSCT is most effective for people who have highly active relapsing MS. That means they're experiencing relapses despite taking one of the more effective DMTs available. It can also help some people with early progressive MS, if they're still experiencing active inflammation.
3. How effective is HSCT for MS?
HSCT has proven to be very effective for people with highly active MS. It can reduce relapses and stabilise or even improve disability for some.
But HSCT can’t regrow nerves or repair damaged myelin. So it can’t help those with advanced progressive MS who are no longer having relapses and don’t shown signs of inflammation on an MRI.
4. What are the risks of HSCT?
Chemotherapy - part of the HSCT procedure - can cause hair loss, fever, nausea and infertility. Your risk of infections in the future also increases. If you already have a lot of nerve damage, as in progressive MS, the chemotherapy can do more harm than good.
Some people have died as a result of HSCT. Since 2005, 1 person in about every 330 who have HSCT have died because of it. This risk can increase for people who are older, have a higher EDSS score, or have certain other conditions. A clinic offering HSCT should be able to explain your own risk, which will depend on lots of things, like your age and overall health.
5. Where can I get HSCT?
HSCT is available on the NHS at limited sites across the UK, but not as a first line treatment. You'll need to be referred by your neurologist or GP. If you're considering getting HSCT outside of the NHS we recommend you check out the credentials of any centre offering the treatment. In Europe such clinics should have JACIE accreditation.
> Find out about JACIE accreditation on the EBMT website
6. Will HSCT become available more widely for MS?
The current eligibility criteria are based on clinical trials that show HSCT can stop active inflammation. But because it's a very intensive treatment it's unlikely to be offered to people whose MS is being controlled by drug DMTs, which come with fewer risks.
Clinical trials of HSCT for advanced MS have not been as successful. This is likely because the worsening of disability results from damage to or loss of nerve cells, rather than through immune attacks.
7. What stem cell treatments are there for MS?
HSCT is the only proven stem cell treatment for MS. Other forms of experimental stem cell therapy should only be offered as part of a regulated clinical trial.
There’s some evidence to suggest mesenchymal stem cell therapy (MSCT) can potentially boost myelin repair and modify the immune system.
Initial results of a phase 2 clinical trial into MSCT suggested it didn’t reduce inflammation any more than a placebo. But scientists are still looking at the data to see if the treatment had other positive outcomes, like repairing myelin.
We updated this blog on Tuesday 21 September 2021